A recent movement has emerged in nutrition and health, under the auspice, ‘Health at Every Size’. Its central tenet is advocating a move toward a “weight-normative” or “weight-inclusive” approach in healthcare, given much recent evidence shows that weight per se is not directly tied to risk for chronic lifestyle disease [1]. These arguments have provoked some thought in nutrition and health research, and in healthcare. We have developed a presumption that weight alone is a determinant of health, but “weight” is not how risk is assessed in healthcare or subjects classified for research purposes: Body Mass Index [BMI] is. At the root of this issue is classification of risk and health status by reference to BMI alone, and researchers arguing for a weight-inclusive paradigm have called for an end to BMI-based decisions in healthcare [2].
Body Mass Index is Redundant
There is merit to this argument. As a clinical tool, BMI lacks strong predictability for health outcomes. Type-2 Diabetes [T2DM] serves as a good example of this issue. While there is an association between BMI and risk for the disease, and increased adiposity does increase risk, the association is not exclusively linear [3]. Defined by BMI alone, adiposity in T2DM varies significantly at time of diagnosis, which indicates that the risk for T2DM is more associated with the underlying metabolic complications that are hallmarks for the disease [4]. Adiposity is a factor in those underlying metabolic complications – impaired fasting blood glucose, insulin resistance, impaired pancreatic beta-cell function – but in analysis of initially healthy subjects with the same baseline BMI, subjects who progressed to diagnoses at 13-years follow-up were those who had greater impairment of those factors when assessed at baseline [5]. Of particular note was the fact that increasing BMI did not increase risk in subjects in this cohort: those who maintained BMI with the ‘overweight’ classification [25-29.9kg/m2] had a higher prevalence of progression to diagnosis and greater declines in pancreatic beta-cell function and insulin sensitivity 5-years prior to diagnosis, compared to persistently obese or progressive weight gaining subjects [4]. Collectively, the data in diabetes shows that underlying metabolic dysfunction is the real driver of disease risk, not BMI.
In addition, BMI does not account for cardiorespiratory fitness, and a phenotype of “fat but fit” has emerged in the literature. There is a continuum of factors influencing degrees of fatness vs. degree of fitness, which interplay to influence the health status of an individual irrespective of the degree of visible adiposity [1]. Assessing health by reference to adiposity alone can thus mislead healthcare practitioners toward incorrect assumptions regarding the health status of the individual based on observed adiposity only, then quantified according to BMI [1].
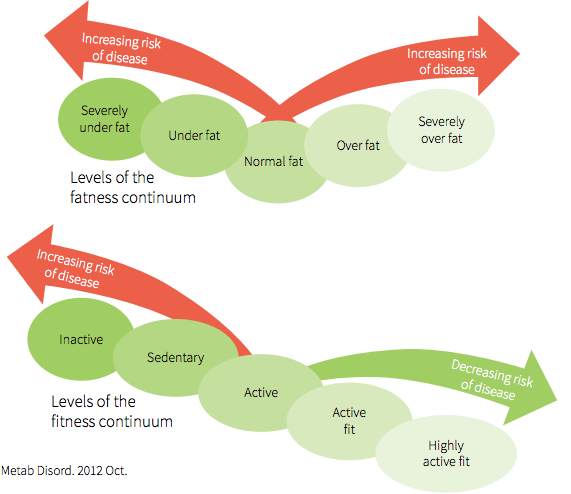 Illustration from: 1, JE. Journal of Diabetes & Metabolic Disorders, 2012; 11:19.
Illustration from: 1, JE. Journal of Diabetes & Metabolic Disorders, 2012; 11:19.
Physical activity and fitness may improve a wide range of physiological factors associated with adiposity, including increasing insulin sensitivity, reducing inflammation, and improving body composition [reduction in fat mass, increased fat-free mass] [1]. In this respect, low levels of cardiorespiratory fitness [CRF], an objective and reliable measure of habitual physical activity levels, are associated with significant increases in risk for cardiometabolic disease [6]. An early analysis of cardiometabolic disease risk associated with fitness compared to BMI found an almost equal relative risk increase from being unfit in both ‘normal’ BMI subjects [19-25kg/m2], and subjects with BMI >27.8kg/m2 [7].
How might fitness attenuate the negative effects of increased adiposity? Within the continuum of “fat-fit”, there are contributing factors to each: cardiorespiratory fitness, musculoskeletal fitness, metabolic flexibility contributing to overall fitness, total fat mass, fat-free mass, and visceral fat contributing to overall adiposity [1]. Counteracting the negative effects of increased adiposity through increased fitness is very much a function of where body composition changes occur.
Waist Circumference: More Predictive of Risk than BMI
I emphasised visible in relation to adiposity above for a particular reason. More recent evidence has emphasised the importance of regional distribution of adipose tissue. An interesting observation in relation to risk for disease and anthropometric risk factors has been that abdominal adiposity, defined by waist-to-hip ratio and waist-to-thigh ratio, is more predictive of cardiometabolic disease than BMI [6].
The relationship between increased CRF and reduced risk for cardiometabolic disease, even at higher levels of adiposity, may be due to positive effects on abdominal adiposity. In analysis of the association between physical activity and cardiometabolic risk, increased physical activity was associated with reduced disease risk over 5.6 years independent of changes in adiposity (8). However, after adjusting for changes in physical activity, energy expenditure, and aerobic fitness, the strongest factor associated with reduced metabolic risk was reductions in waist circumference [8].
This may be more indicative of the beneficial effects of reducing central, abdominal – i.e., visceral – adiposity. While associations between BMI and adverse health outcomes are typically consistent at higher obesity classifications [>30kg/m2], waist-hip ratio has been shown to be predictive of risk amongst both persons with obesity [BMI >30kg/m2] and persons defined as ‘normal’ weight [BMI 19-25kg/m2]. This association has been confirmed by meta-analysis of recent studies utilizing imaging techniques to assess visceral fat accumulation: visceral fat is strongly predictive of adverse cardiometabolic health outcomes, yet reductions in visceral and total abdominal fat may occur without any change in BMI [9].
Thus, while CRF is important, it is arguable that the benefit of increased fitness is a result of body composition changes in specific tissues: in particular a reduction in visceral abdominal fat, and increased metabolic flexibility in skeletal muscle [1]. Thus, a simple assessment of weight status like BMI fails to give any true assessment of individual health status, which is influenced by this interaction between body composition and adipose tissue distribution.
All Fat is Not Created Equally
The phenomenon of “fat but fit” or “healthy obese” implies a dichotomy: that ‘metabolic obesity,’ i.e. the negative effects of increased adiposity, is a consequence of adipose tissue function. The dated view of adipose tissue was as a benign, storage depot. Adipose tissue is now recognized as an endocrine and immunoregulatory organ, responsible for the synthesis and secretion of signaling compounds involved in energy balance, substrate metabolism, inflammation, and immunity [10]. Obese but metabolically healthy subjects are defined by low hepatic triglyceride [TG] levels and normal insulin sensitivity, and demonstrate resistance to adverse cardiometabolic effects from increases in adiposity, compared to obese but metabolically unhealthy subjects [11].
What underlies these differences is the variance in metabolic effects of adipose tissues. In metabolically unhealthy obesity, the function of adipocytes is compromised, resulting in an overspill of triglycerides to other tissues, in particular the liver [10]. The accumulation of triglycerides in the liver upregulates VLDL [very-low density lipoprotein] synthesis and contributes to remodelling of LDL-C and HDL-C [12]. The resulting “atherogenic lipoprotein phenotype,” defined by high LDL, low HDL, and high triglycerides, is the most significant risk factor for cardiovascular disease [12]. The increase in liver fat deposition also drives hepatic insulin resistance, causing increased concentrations of circulating free fatty acids in the postprandial period, contributing to new TG synthesis and impairing postprandial TG clearance [13][14]. The increase in flow of fats to other tissues, known as ‘lipotoxicity,’ in turn drives increased adipose tissue inflammatory signalling, immune responses, and oxidative stress [10].
The primary driver of these negative effects is certainly increasing adiposity, but more particularly the how and where of adipocyte hypertrophy [10]. In this regard, it is important to note that there are two mechanisms of adipocyte enlargement which strongly influence obesity risk: hyperplastic obesity and hypertrophic obesity [15]. Hypertrophic obesity is defined by increasing size of existing adipocytes; this is an issue as the ability to store triglycerides becomes compromised [picture a balloon getting too full], and the rate at which adiposity is increasing is greater than the capacity of the body to create new adipocytes to store excess energy in [15]. The result is a spillover of free fatty acids [FFA] into circulation, which are then disposed to other body organs and tissues [10]. In contrast, hyperplastic obesity is defined by small adipocytes, but an increased number of total cells that maintain insulin sensitivity through intact GLUT4 translocation and have low levels of inflammatory cytokine secretion [15]. The variance between the two is highly relevant to the associations between central abdominal adiposity and cardiometabolic risk elucidated above. Abdominal adipocytes are more hypertrophic, and thus more likely to contribute to FFA spillover and associated lipotoxicity [16]. In contrast, subcutaneous adipocytes are smaller, more numerous, and thus have increased capacity to remove FFA from circulation, preventing lipotoxicity [16].
Thus, the distribution and type of adipose tissue has been identified as the primary factor influencing metabolic health, independent of total adiposity. In humans, subcutaneous adipose tissue [SAT] constitutes >80% of total body fat, and is highly concentrated in the abdominal, gluteal and femoral regions [17]. Intra-abdominal adipose tissue [IAAT] accounts for 10-20% of total fat in men and 5-10% in women and is associated with internal organs, in particular, digestive organs and associated visceral fat [17].
Of the various fat depots, the strongest associations with adverse cardiometabolic effects are observed with increased subcutaneous abdominal adipose tissue [SAAT] and visceral adipose tissue [VAT] [18]. However, in assessing risk as a function of adipose tissue distribution, an interesting observation has emerged: that both adipocyte type [hypertrophic vs. hyperplastic] and distribution differ by sex [19]. Men and women have differential levels of total body fat and differences in distribution, which influences differences in metabolic health as a consequence of adiposity [19].
Sex Dimorphism and Adipose Tissue Distribution
Women have a higher percentage of total body fat than men, a characteristic that develops through puberty, and with divergent regional storage: men preferentially increase VAT stores, while women predominantly accumulate SAT [20]. This differential fat deposition is evident from puberty: men distribute more adipose tissue in the central abdominal region, while women are characterised by gluteal-femoral adipose deposition [21].
The primary driver of this sex dimorphism are sex steroid hormones. Under the influence of oestrogens, women preferentially shift fat deposition toward SAT accumulation in the gluteal-femoral region and are protected against VAT accumulation [22]. The prominent role of oestrogens influencing adipose tissue deposition is supported by the marked shift in distribution which occurs in post-menopausal women, from SAT accumulation to increased IAAT and VAT deposition [19]. This is attributed to the fact that oestrogen production shifts in the postmenopausal period from ovarian oestrogen production to adipose tissue and the adrenal glands, and conversion of oestrogen precursors occurs via aromatisation in visceral adipose tissues [16]. It is hypothesised that the preferential partitioning of fat into subcutaneous depots reflects an evolutionary selection bias to support the additional energetic requirements of lactation [16].
In contrast, androgens are implicated in the accumulation of abdominal adiposity and VAT in particular, a role which is also supported by the increases in VAT associated with declining testosterone levels in ageing men [23]. In addition, women with androgen-dominant polycystic ovarian syndrome [PCOS] are also characterised by central adiposity, and associated insulin resistance and cardiometabolic risk factors [23]. Testosterone has been shown to have an inhibitory effect on lipoprotein lipase [LPL] activity in femoral subcutaneous adipocytes in men [24]. Visceral adipocytes also have high FFA turnover and provide a rapid delivery route for FFA to the liver via the hepatic portal vein, which is hypothesised to reflect an evolutionary adaptation in men for swift mobilisation of energy required on extended hunting expeditions [16].
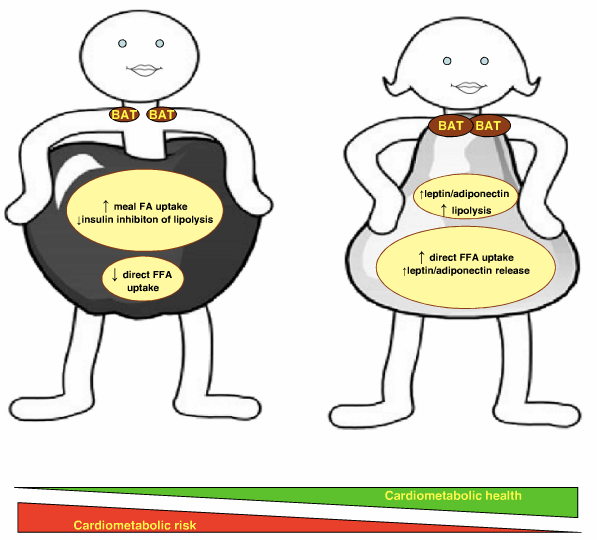 Illustration from: Karastergiou, K., Smith, S., Greenberg, A. and Fried, S. (2012). Sex differences in human adipose tissues: the biology of pear shape. Biology of Sex Differences, 3(1), p.13.
Illustration from: Karastergiou, K., Smith, S., Greenberg, A. and Fried, S. (2012). Sex differences in human adipose tissues: the biology of pear shape. Biology of Sex Differences, 3(1), p.13.
Whatever the evolutionary underpinnings of sex dimorphism in adipose tissue distribution, cardiometabolic risk in the modern world ties strongly to regional deposition. In general, pre-menopausal women have a reduced overall risk of cardiometabolic disease compared to men, a fact that is associated with the preferential distribution of fat into SAT in the gluteal-femoral region [19]. For example, in the famous Framingham Heart Study, increasing VAT linearly increased risk for cardiometabolic risk factors, and this effect was more strongly associated with adverse health outcomes in women who increased VAT [25]. In general, when matched for weight and age, men have higher rates of cardiovascular disease than women [10]. An understanding of the underlying mechanisms influencing sex differences in cardiometabolic disease risk is thus warranted.
Mechanisms
The distribution of adipose tissue influences multiple factors, including inflammation, insulin sensitivity, adipokines, free fatty acid [FFA] release, lipogenesis and lipolysis [26]. The IAAT fat depot in particular contributes to impaired glucose tolerance and is more predictive of cardiometabolic risk than basic anthropometric measurements [25][27][28]. Men are noted to have significantly greater deposition of fat in IAAT than women, and high levels of IAAT contribute to insulin resistance, and suppression of lipolysis [29]. However, while insulin resistance suppresses lipolysis in IAAT, insulin does not have this effect on VAT; visceral fat cells continue to release FFA despite high levels of insulin [29] [aside: another nail in the coffin for the carb-insulin hypothesis is the continued release of FFA in the presence of hyperinsulinemia]. The high rates of lipolysis observed in VAT lead to continued elevations in circulating FFA, resulting in hepatic insulin resistance, high blood glucose, high triglycerides, and lipoprotein remodelling: a clustering of cardiometabolic risk factors [13].
However, in women, the gluteal-femoral region is associated with hyperplastic adipocytes, and the increased number of adipocytes results in greater capacity to store fat in lower body subcutaneous tissue [30]. The gluteal-femoral region is associated with reduced adipocyte turnover of FFA and reduced utilisation, which taken together with the increased capacity of gluteal-femoral SAT to store fat, prevents FFA from accumulating in visceral depots, in contrast to men [30]. The gluteal-femoral region thus acts as a “metabolic sink” for excess FFA and protects against the adverse cardiometabolic effects of high circulating FFA levels [31]. The increased capacity of gluteal-femoral SAT for FFA uptake in women with lower-body adiposity is in direct contrast to the increased accumulation of FFA in visceral depots in men [32]. The difference in effects of adipose tissue depot is evident across the sexes, however, as women with upper body adiposity display the same metabolic complications as men, influenced by the increased release of FFA from upper body depots [33]. In a comparison between women with lower or upper body adiposity, women with lower body deposition displayed less arterial calcification, a marker of cardiovascular disease, than women with upper body deposition [34].
In contrast, central VAT in men has pronounced deleterious effects on inflammation and lipid profiles, both cardiovascular risk factors [35]. Increasing VAT stores is more strongly associated with increased inflammatory cytokine signalling, while SAT depots have a reduced inflammatory profile [17]. Gluteal-femoral SAT is not associated with high levels of inflammatory signalling [36]. There are also marked differences in adipokine secretions, with gluteal-femoral SAT associated with preservation of circulating leptin and adiponectin levels, which regulate energy balance and substrate metabolism [36]. In fact, the interaction between adipose deposition and endocrine function is a primary feature of sex dimorphism. In particular, the preservation of adiponectin levels with gluteal-femoral SAT may be a primary factor influencing protective cardiometabolic effects. Adiponectin is derived from adipocytes and exhibits sex dimorphism with higher levels in women than men [37]. Adiponectin increases insulin sensitising and enhances glucose uptake, which may contribute to preserving insulin sensitivity in gluteal-femoral SAT in women [38]. In addition, adiponectin increases FFA oxidation and is inversely associated with VAT, protecting against central adiposity and circulating FFA [36]. In addition, a feature of expanding hypertrophic adipose tissue is infiltration with inflammatory and immune cells [37]. Hyperplastic adipose tissue expansion preserves adiponectin levels, in turn protecting against inflammatory and immune responses [37]. A particularly important point in this respect is the decline in adiponectin with increasing adiposity [37]. Increased hypertrophic adiposity in men, with concomitant reductions in adiponectin [already lower in men than women], may thus exacerbate the low-grade systemic inflammation and immune activation noted with obesity [37][38].
Another notable difference in adipose tissue functions between the sexes is in the metabolic activity of brown adipose tissue [BAT], which is enhanced under the influence of oestrogen [16]. Women naturally display higher BAT levels than men, and the capacity for oestrogen to modulate ‘browning’ of white adipose tissue [WAT] may confer protection against increasing adiposity through a combination of increased metabolic activity of adipose tissue, and reduced appetite [39]. BAT displays less inflammatory signalling than WAT and may have an insulin-sensitising effect [40]. While research surrounding the influence of BAT on health and adiposity is still emerging, the increased levels of BAT in women may be another mechanism through which adverse cardiometabolic outcomes are attenuated.
Conclusions
The current assessment of health status by reference to visual adiposity or basic anthropometric assessments like BMI does little to reflect the underlying metabolic complications associated with adipose tissue function. This can create incorrect assumptions regarding health status, without regard for objective risk factors. The sex dimorphism in adipose tissue function, defined by higher central IAAT and VAT deposition in men, and greater gluteal-femoral SAT deposition in women, is more than a benign difference in regional distribution: it directly correlates to underlying metabolic complications resulting from the differing activity of adipose tissue depots.
Gluteal-femoral SAT displays significant differences in FFA storage capacity, insulin sensitivity, adiponectin preservation, and inflammatory and endocrine signalling, which collectively favour a protective effect of this adipose tissue depot on cardiometabolic risk factors. In contrast, central adiposity and increases in VAT linearly increase cardiometabolic risks independent of BMI, including insulin resistance, dyslipidemia, and inflammation.
Defined by BMI, a ‘normal’ weight man could potentially be passed over as ‘healthy,’ while an ‘overweight’ women could be incorrectly considered at risk of adverse health outcomes. However, going beyond BMI, that same ‘normal’ weight man could present with high waist circumference, high triglycerides, and abnormal lipoprotein levels: he is thus a significant cardiometabolic risk. Conversely, the same ‘overweight’ women could have a low waist-to-hip ratio, normal blood lipids, and normal glucose tolerance: she is thus objectively metabolically healthy. The prescription of weight loss in the latter is unethical; in the former it’s a necessity. BMI does not allow for an objective quantification of metabolic risk.
These factors are crucial considerations in the assessment and treatment of obesity and warrant a sex-specific evaluation of risk by reference to objective risk factors.
References
- Clark, J. (2012). An overview of the contribution of fatness and fitness factors, and the role of exercise, in the formation of health status for individuals who are overweight. Journal of Diabetes & Metabolic Disorders, 11(19).
- Tylka, T., Annunziato, R., Burgard, D., DanÃelsdóttir, S., Shuman, E., Davis, C. and Calogero, R. (2014). The Weight-Inclusive versus Weight-Normative Approach to Health: Evaluating the Evidence for Prioritizing Well-Being over Weight Loss. Journal of Obesity, 2014, pp.1-18.
- Abdullah, A., Peeters, A., de Courten, M. and Stoelwinder, J. (2010). The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Research and Clinical Practice, 89(3), pp.309-319.
- Vistisen, D., Witte, D., Tabek, A., Herder, C., Brunner, E., Kivimaki, M. and Farch, K. (2014). Patterns of Obesity Development before the Diagnosis of Type 2 Diabetes: The Whitehall II Cohort Study. PLoS Medicine, 11(2), p.e1001602.
- Tabek, A., Jokela, M., Akbaraly, T., Brunner, E., Kivimaki, M. and Witte, D. (2009). Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. The Lancet, 373(9682), pp.2215-2221.
- Hainer, V., Toplak, H. and Stich, V. (2009). Fat or Fit: What Is More Important?. Diabetes Care, 32(suppl_2), pp.S392-S397.
- Ekelund, U., Brage, S., Franks, P., Hennings, S., Emms, S. and Wareham, N. (2005). Physical Activity Energy Expenditure Predicts Progression Toward the Metabolic Syndrome Independently of Aerobic Fitness in Middle-Aged Healthy Caucasians: The Medical Research Council Ely Study. Diabetes Care, 28(5), pp.1195-1200.
- Kay, S. and Fiatarone Singh, M. (2006). The influence of physical activity on abdominal fat: a systematic review of the literature. Obesity Reviews, 7(2), pp.183-200.
- Lee, C., Jackson, A. and Blair, S. (1998). US weight guidelines: is it also important to consider cardiorespiratory fitness?. Int J Obes Relat Metab Disord, 1998(22), pp.S2-27.
- Bays, HE. (2011). Journal of the American College of Cardiology, 57(25), pp.2461-2473.
- Fabbrini, E., Yoshino, J., Yoshino, M., Magkos, F., Tiemann Luecking, C., Samovski, D., Fraterrigo, G., Okunade, A., Patterson, B. and Klein, S. (2015). Metabolically normal obese people are protected from adverse effects following weight gain. Journal of Clinical Investigation, 125(2), pp.787-795
- Griffin, B. (2013). Lipid Metabolism. Surgery, 31(6), pp.267-272.
- Kotronen, A. and Yki-Jarvinen, H. (2007). Fatty Liver: A Novel Component of the Metabolic Syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(1), pp.27-38.
- Parks, E. (2002). Changes in fat synthesis influenced by dietary macronutrient content. Proceedings of the Nutrition Society, 61(02), pp.281-286.
- Choe, S., Huh, J., Hwang, I., Kim, J. and Kim, J. (2016). Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Frontiers in Endocrinology, 7.
- Palmer, B. and Clegg, D. (2015). The sexual dimorphism of obesity. Mol Cell Endocrinol, Feb 15(0), pp.113–119.
- Lee, M., Wu, Y. and Fried, S. (2013). Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Molecular Aspects of Medicine, 34(1), pp.1-11.
- Smith, S., Lovejoy, J., Greenway, F., Ryan, D., deJonge, L., de la Bretonne, J., Volafova, J. and Bray, G. (2001). Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism, 50(4), pp.425-435.
- White, U. and Tchoukalova, Y. (2014). Sex dimorphism and depot differences in adipose tissue function. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, 1842(3), pp.377-392.
- Gallagher, D., Visser, M., Sepulveda, D., Pierson, R., Harris, T. and Heymsfield, S. (1996). How Useful Is Body Mass Index for Comparison of Body Fatness across Age, Sex, and Ethnic Groups?. American Journal of Epidemiology, 143(3), pp.228-239.
- Camhi, S., Bray, G., Bouchard, C., Greenway, F., Johnson, W., Newton, R., Ravussin, E., Ryan, D., Smith, S. and Katzmarzyk, P. (2011). The Relationship of Waist Circumference and BMI to Visceral, Subcutaneous, and Total Body Fat: Sex and Race Differences. Obesity, 19(2), pp.402-408.
- Shi, H. and Clegg, D. (2009). Sex differences in the regulation of body weight. Physiol. Behav, 97(2009), pp.pp. 199–204.
- Karastergiou, K., Smith, S., Greenberg, A. and Fried, S. (2012). Sex differences in human adipose tissues: the biology of pear shape. Biology of Sex Differences, 3(1), p.13.
- Ramirez, M., McMurray, M., Wiebke, G., Felten, K., Ren, K. and Meikle, A. (1997). Evidence for sex steroid inhibition of lipoprotein lipase in men: comparison of abdominal and femoral adipose tissue. Metabolism, 1997(46), pp.179-185.
- Fox, C., Massaro, J., Hoffmann, U., Pou, K., Maurovich-Horvat, P., Liu, C., Vasan, R., Murabito, J., Meigs, J., Cupples, L., D’Agostino, R. and O’Donnell, C. (2007). Abdominal Visceral and Subcutaneous Adipose Tissue Compartments: Association With Metabolic Risk Factors in the Framingham Heart Study. Circulation, 116(1), pp.39-48.
- Fuente-Martin, E., Argente-Arizan, P., Ros, P., Argente, J. and Chowen, J. (2013). Sex differences in adipose tissue. Adipocyte, 2(3), pp.128-134.
- Thomas, E., Parkinson, J., Frost, G., Goldstone, A., Dorac, C., McCarthy, J., Collins, A., Fitzpatrick, J., Durighel, G., Taylor-Robinson, S. and Bell, J. (2011). The Missing Risk: MRI and MRS Phenotyping of Abdominal Adiposity and Ectopic Fat. Obesity, 20(1), pp.76-87.
- Ross, R., Aru, J., Freeman, J., Hudson, R. and Janssen, I. (2002). Abdominal adiposity and insulin resistance in obese men. American Journal of Physiology – Endocrinology And Metabolism, 282(3), pp.E657-E663.
- Nielsen, S., Guo, Z., Johnson, C., Hensrud, D. and Jensen, M. (2004). Splanchnic lipolysis in human obesity. Journal of Clinical Investigation, 113(11), pp.1582-1588.
- Bloor, I. and Symonds, M. (2014). Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Hormones and Behavior, 66(1), pp.95-103.
- Lemieux, I. (2004). Energy Partitioning in Gluteal-Femoral Fat: Does the Metabolic Fate of Triglycerides Affect Coronary Heart Disease Risk?. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(5), pp.795-797.
- Shadid, S., Koutsari, C. and Jensen, M. (2007). Direct Free Fatty Acid Uptake Into Human Adipocytes In Vivo: Relation to Body Fat Distribution. Diabetes, 56(5), pp.1369-1375.
- Jensen, M. (2008). Role of Body Fat Distribution and the Metabolic Complications of Obesity. The Journal of Clinical Endocrinology & Metabolism, 93(11_supplement_1), pp.s57-s63.
- Tanko, L. (2003). Central and peripheral fat mass have contrasting effect on the progression of aortic calcification in postmenopausal women. European Heart Journal, 24(16), pp.1531-1537.
- Abraham, T., Pedley, A., Massaro, J., Hoffmann, U. and Fox, C. (2015). Association Between Visceral and Subcutaneous Adipose Depots and Incident Cardiovascular Disease Risk FactorsCLINICAL PERSPECTIVE. Circulation, 132(17), pp.1639-1647.
- Manolopoulos, K., Karpe, F. and Frayn, K. (2010). Gluteofemoral body fat as a determinant of metabolic health. International Journal of Obesity, 34(6), pp.949-959.
- Nigro, E., Scudiero, O., Monaco, M., Palmieri, A., Mazzarella, G., Costagliola, C., Bianco, A. and Daniele, A. (2014). New Insight into Adiponectin Role in Obesity and Obesity-Related Diseases. BioMed Research International, 2014, pp.1-14.
- Bidulescu, A., Liu, J., Hickson, D., Hairston, K., Fox, E., Arnett, D., Sumner, A., Taylor, H. and Gibbons, G. (2013). Gender differences in the association of visceral and subcutaneous adiposity with adiponectin in African Americans: the Jackson Heart Study. BMC Cardiovascular Disorders, 13(1).
- Nookaew, I., Svensson, P., Jacobson, P., Jernas, M., Taube, M., Larsson, I., Andersson-Assarsson, J., Sjastram, L., Froguel, P., Walley, A., Nielsen, J. and Carlsson, L. (2013). Adipose Tissue Resting Energy Expenditure and Expression of Genes Involved in Mitochondrial Function Are Higher in Women than in Men. The Journal of Clinical Endocrinology & Metabolism, 98(2), pp.E370-E378.
- Villarroya, J., Cereijo, R. and Villarroya, F. (2013). An endocrine role for brown adipose tissue?. AJP: Endocrinology and Metabolism, 305(5), pp.E567-E572.




